Theranostics can treat stage 4 prostate cancer.
Theranostics is designed for men diagnosed with advanced prostate cancer, metastatic prostate cancer, or stage 4 prostate cancer.


Theranostics is not chemotherapy.
Theranostics is a non-invasive, targeted therapy that uses advanced molecular imaging and precision oncology to attack cancer cells without the harsh side effects commonly associated with chemotherapy.
Theranostics is a non-invasive, targeted therapy that uses advanced molecular imaging and precision oncology to attack cancer cells without the harsh side effects commonly associated with chemotherapy.
How does Theranostics work?
Theranostics uses specialized molecules to diagnose and treat cancer, offering a more targeted and effective treatment option.
PLUVICTO is the radiopharmaceutical drug used to treat adults with an advanced cancer called prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC) that:
- has spread to other parts of the body (metastatic), and
- has already been treated with other anticancer treatments

What you need to know.
Who is a candidate for PSMA Theranostics therapy?
Currently, patients with advanced prostate cancer are candidates, after they have received traditional therapies like Androgen Deprivation Therapy and Chemotherapy. Soon, we are expecting Theranostics will be approved so it can be utilized earlier in prostate cancer care.
What is Prostate Cancer?
Prostate cancer is a type of cancer that develops in the prostate gland, which is part of the male reproductive system. It is one of the most common cancers in men. It is the #2 cause of cancer death in men, after lung cancer.
What is PSMA Theranostics therapy?
PSMA Theranostics therapy is a form of Theranostics, specifically used for prostate cancer. It targets the Prostate-SpecificMembrane Antigen (PSMA) present on prostate cancer cells,delivering targeted therapy directly to the cancer. PSMA is overexpressed – up to 1000X more in cancer cells – compared tonormal cells. Targeted therapy can be delivered to the cancer cells, with minimal effect on healthy tissue.
How is PSMA Theranostics therapy administered?
PSMA Theranostics is provided via intravenous injection for both the diagnostic stage and the therapeutic stage. A radioactive drug – called a radiopharmaceutical – travels through the bloodstream and binds to PSMAexpressing cells: prostate cancer cells. During the first stage of treatment, an imaging device like a PET/CT detects the location of the tumors because they are emitting a small amount of radiation, due to the attached radiopharmaceutical. The initial imaging, and its evaluation, is what determines if someone is a candidate for Theranostics. During the second stage of treatment, a different, stronger radiopharmaceutical – PLUVICTO, will attach to the cancer cells and destroy them, leaving healthy cells alone.
Are there any side effects of PSMA Theranostics therapy?
Common side effects may include temporary fatigue, dry mouth, nausea, and mild pain at the injection site.
What type of cancers can be treated with Theranostics?
Currently, Theranostics is FDA approved for prostate cnacer and neuroendocrine tumors.
There is active research currently to bring Theranostics to multiple other solid tumor cancers. We expect, in the foreseeable future, each cancer will have its own unique Theranostics therapy. This will increase patient quality of life and overall outcomes.
Is Theranostics covered by insurance?
Yes, Theranostics is typically covered by insurance for patients with stage 4 or metastatic prostate cancer. Coverage may vary depending on your specific insurance plan, so it’s recommended to check with your provider for details.
Where can I get this treatment?
Privado Health has the following treatment locations:
West Coast
- Las Vegas, NV
- Irvine, California
- Anaheim Hills, California
- Newport Beach, California
- Loma Linda, California
East Coast
- Miami, Florida
International
- Germany
- Turkey
- Australia
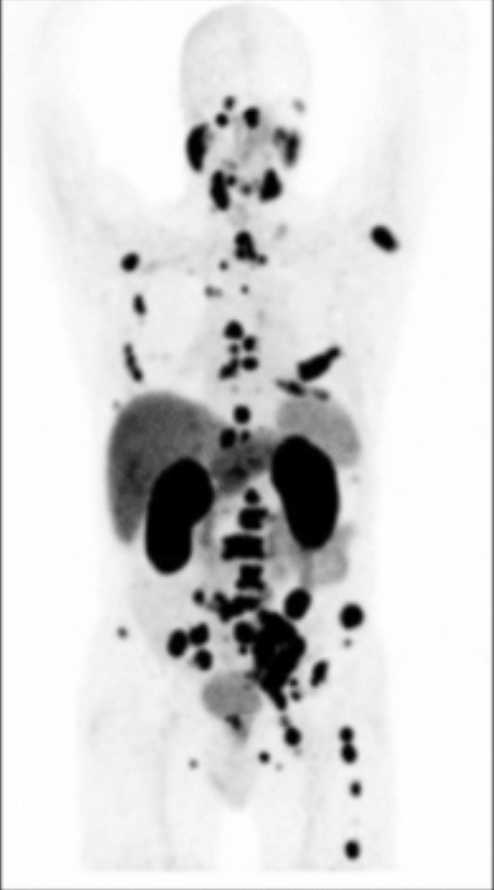
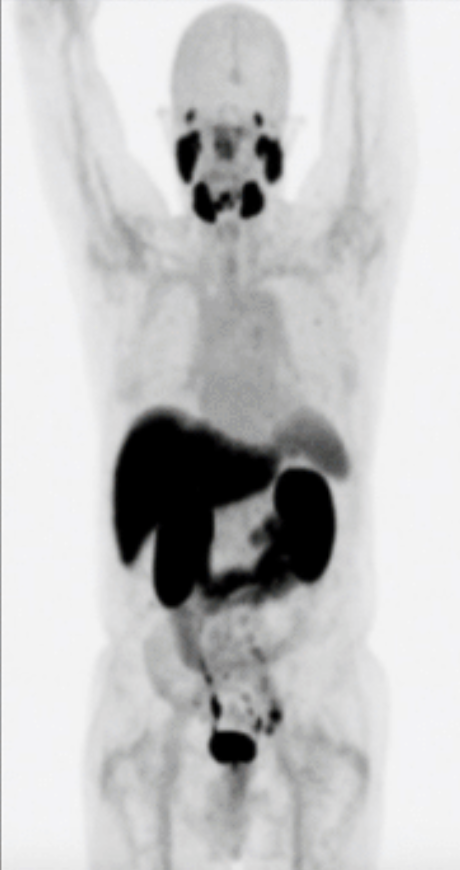

Alan Held eradicated 80% of his prostate cancer with Theranostics.
Alan Held underwent a groundbreaking theranostics treatment for prostate cancer in Germany. This innovative approach, led by Dr. Frankis Almaguel at Loma Linda University Medical Center, eradicated 80% of Held’s cancer cells after just one session. With thousands benefiting from theranostics in Germany, the FDA has now approved Theranostics for use in the U.S., signaling a transformative shift in prostate cancer care. The before and after shows Mr. Held’s results from treatment with Theranostics.


